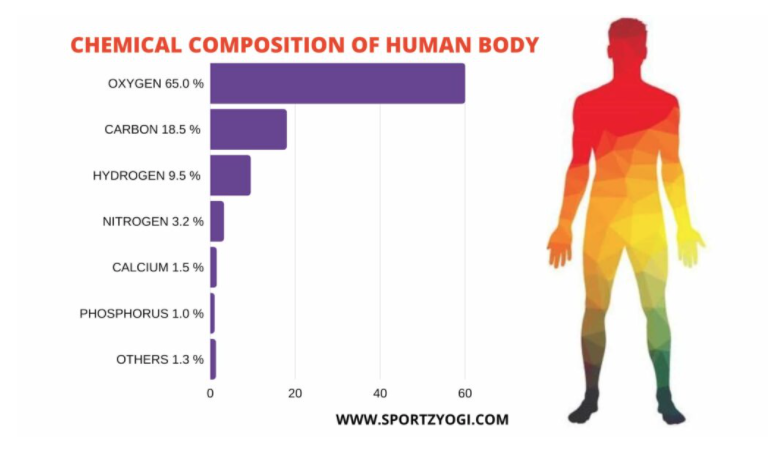
Chemical Composition of Body
Do you know what the smallest thing in the world is? It’s an atom! Atoms are so small that we can’t see them with our eyes. They are the basic building blocks of every solid, liquid, and gas. Four elements—hydrogen, oxygen, carbon, and nitrogen— make up 99% of the atoms in the human body. There are 7 mineral elements in the body, like calcium and phosphorus, which are in your bones. Others include potassium, sulfur, sodium, chlorine, and magnesium. There are 13 trace elements that we need in extremely small amounts but are essential for normal growth and function. One example is iron, which is needed for the blood cells to be able to transport oxygen around your body.
Molecules are two or more atoms bonded together. The atoms can be stuck together in a few different ways. The first way is called a covalent bond, which is the strongest type of bond between atoms. Weaker types of bonds include polar and non-polar covalent bonds. A hydrogen bond is a very weak electrical attraction. Water makes up 99% of all molecules in the body and it is also known as H2O. it is made up of 2 polar covalent bonds (between oxygen and the two hydrogens within the water molecule) and hydrogen bonds (between different water molecules). The body has 3 classes of organic molecules including carbohydrates, lipids, proteins, and nucleic acids, which we will go over in the next article.
Picture Source: sportzyogi.com